License
Attribution-ShareAlike 4.0 International
=======================================================================
Creative Commons Corporation ("Creative Commons") is not a law firm and
does not provide legal services or legal advice. Distribution of
Creative Commons public licenses does not create a lawyer-client or
other relationship. Creative Commons makes its licenses and related
information available on an "as-is" basis. Creative Commons gives no
warranties regarding its licenses, any material licensed under their
terms and conditions, or any related information. Creative Commons
disclaims all liability for damages resulting from their use to the
fullest extent possible.
Using Creative Commons Public Licenses
Creative Commons public licenses provide a standard set of terms and
conditions that creators and other rights holders may use to share
original works of authorship and other material subject to copyright
and certain other rights specified in the public license below. The
following considerations are for informational purposes only, are not
exhaustive, and do not form part of our licenses.
Considerations for licensors: Our public licenses are
intended for use by those authorized to give the public
permission to use material in ways otherwise restricted by
copyright and certain other rights. Our licenses are
irrevocable. Licensors should read and understand the terms
and conditions of the license they choose before applying it.
Licensors should also secure all rights necessary before
applying our licenses so that the public can reuse the
material as expected. Licensors should clearly mark any
material not subject to the license. This includes other CC-
licensed material, or material used under an exception or
limitation to copyright. More considerations for licensors:
wiki.creativecommons.org/Considerations_for_licensors
Considerations for the public: By using one of our public
licenses, a licensor grants the public permission to use the
licensed material under specified terms and conditions. If
the licensor's permission is not necessary for any reason--for
example, because of any applicable exception or limitation to
copyright--then that use is not regulated by the license. Our
licenses grant only permissions under copyright and certain
other rights that a licensor has authority to grant. Use of
the licensed material may still be restricted for other
reasons, including because others have copyright or other
rights in the material. A licensor may make special requests,
such as asking that all changes be marked or described.
Although not required by our licenses, you are encouraged to
respect those requests where reasonable. More considerations
for the public:
wiki.creativecommons.org/Considerations_for_licensees
=======================================================================
Creative Commons Attribution-ShareAlike 4.0 International Public
License
By exercising the Licensed Rights (defined below), You accept and agree
to be bound by the terms and conditions of this Creative Commons
Attribution-ShareAlike 4.0 International Public License ("Public
License"). To the extent this Public License may be interpreted as a
contract, You are granted the Licensed Rights in consideration of Your
acceptance of these terms and conditions, and the Licensor grants You
such rights in consideration of benefits the Licensor receives from
making the Licensed Material available under these terms and
conditions.
Section 1 -- Definitions.
a. Adapted Material means material subject to Copyright and Similar
Rights that is derived from or based upon the Licensed Material
and in which the Licensed Material is translated, altered,
arranged, transformed, or otherwise modified in a manner requiring
permission under the Copyright and Similar Rights held by the
Licensor. For purposes of this Public License, where the Licensed
Material is a musical work, performance, or sound recording,
Adapted Material is always produced where the Licensed Material is
synched in timed relation with a moving image.
b. Adapter's License means the license You apply to Your Copyright
and Similar Rights in Your contributions to Adapted Material in
accordance with the terms and conditions of this Public License.
c. BY-SA Compatible License means a license listed at
creativecommons.org/compatiblelicenses, approved by Creative
Commons as essentially the equivalent of this Public License.
d. Copyright and Similar Rights means copyright and/or similar rights
closely related to copyright including, without limitation,
performance, broadcast, sound recording, and Sui Generis Database
Rights, without regard to how the rights are labeled or
categorized. For purposes of this Public License, the rights
specified in Section 2(b)(1)-(2) are not Copyright and Similar
Rights.
e. Effective Technological Measures means those measures that, in the
absence of proper authority, may not be circumvented under laws
fulfilling obligations under Article 11 of the WIPO Copyright
Treaty adopted on December 20, 1996, and/or similar international
agreements.
f. Exceptions and Limitations means fair use, fair dealing, and/or
any other exception or limitation to Copyright and Similar Rights
that applies to Your use of the Licensed Material.
g. License Elements means the license attributes listed in the name
of a Creative Commons Public License. The License Elements of this
Public License are Attribution and ShareAlike.
h. Licensed Material means the artistic or literary work, database,
or other material to which the Licensor applied this Public
License.
i. Licensed Rights means the rights granted to You subject to the
terms and conditions of this Public License, which are limited to
all Copyright and Similar Rights that apply to Your use of the
Licensed Material and that the Licensor has authority to license.
j. Licensor means the individual(s) or entity(ies) granting rights
under this Public License.
k. Share means to provide material to the public by any means or
process that requires permission under the Licensed Rights, such
as reproduction, public display, public performance, distribution,
dissemination, communication, or importation, and to make material
available to the public including in ways that members of the
public may access the material from a place and at a time
individually chosen by them.
l. Sui Generis Database Rights means rights other than copyright
resulting from Directive 96/9/EC of the European Parliament and of
the Council of 11 March 1996 on the legal protection of databases,
as amended and/or succeeded, as well as other essentially
equivalent rights anywhere in the world.
m. You means the individual or entity exercising the Licensed Rights
under this Public License. Your has a corresponding meaning.
Section 2 -- Scope.
a. License grant.
1. Subject to the terms and conditions of this Public License,
the Licensor hereby grants You a worldwide, royalty-free,
non-sublicensable, non-exclusive, irrevocable license to
exercise the Licensed Rights in the Licensed Material to:
a. reproduce and Share the Licensed Material, in whole or
in part; and
b. produce, reproduce, and Share Adapted Material.
2. Exceptions and Limitations. For the avoidance of doubt, where
Exceptions and Limitations apply to Your use, this Public
License does not apply, and You do not need to comply with
its terms and conditions.
3. Term. The term of this Public License is specified in Section
6(a).
4. Media and formats; technical modifications allowed. The
Licensor authorizes You to exercise the Licensed Rights in
all media and formats whether now known or hereafter created,
and to make technical modifications necessary to do so. The
Licensor waives and/or agrees not to assert any right or
authority to forbid You from making technical modifications
necessary to exercise the Licensed Rights, including
technical modifications necessary to circumvent Effective
Technological Measures. For purposes of this Public License,
simply making modifications authorized by this Section 2(a)
(4) never produces Adapted Material.
5. Downstream recipients.
a. Offer from the Licensor -- Licensed Material. Every
recipient of the Licensed Material automatically
receives an offer from the Licensor to exercise the
Licensed Rights under the terms and conditions of this
Public License.
b. Additional offer from the Licensor -- Adapted Material.
Every recipient of Adapted Material from You
automatically receives an offer from the Licensor to
exercise the Licensed Rights in the Adapted Material
under the conditions of the Adapter's License You apply.
c. No downstream restrictions. You may not offer or impose
any additional or different terms or conditions on, or
apply any Effective Technological Measures to, the
Licensed Material if doing so restricts exercise of the
Licensed Rights by any recipient of the Licensed
Material.
6. No endorsement. Nothing in this Public License constitutes or
may be construed as permission to assert or imply that You
are, or that Your use of the Licensed Material is, connected
with, or sponsored, endorsed, or granted official status by,
the Licensor or others designated to receive attribution as
provided in Section 3(a)(1)(A)(i).
b. Other rights.
1. Moral rights, such as the right of integrity, are not
licensed under this Public License, nor are publicity,
privacy, and/or other similar personality rights; however, to
the extent possible, the Licensor waives and/or agrees not to
assert any such rights held by the Licensor to the limited
extent necessary to allow You to exercise the Licensed
Rights, but not otherwise.
2. Patent and trademark rights are not licensed under this
Public License.
3. To the extent possible, the Licensor waives any right to
collect royalties from You for the exercise of the Licensed
Rights, whether directly or through a collecting society
under any voluntary or waivable statutory or compulsory
licensing scheme. In all other cases the Licensor expressly
reserves any right to collect such royalties.
Section 3 -- License Conditions.
Your exercise of the Licensed Rights is expressly made subject to the
following conditions.
a. Attribution.
1. If You Share the Licensed Material (including in modified
form), You must:
a. retain the following if it is supplied by the Licensor
with the Licensed Material:
i. identification of the creator(s) of the Licensed
Material and any others designated to receive
attribution, in any reasonable manner requested by
the Licensor (including by pseudonym if
designated);
ii. a copyright notice;
iii. a notice that refers to this Public License;
iv. a notice that refers to the disclaimer of
warranties;
v. a URI or hyperlink to the Licensed Material to the
extent reasonably practicable;
b. indicate if You modified the Licensed Material and
retain an indication of any previous modifications; and
c. indicate the Licensed Material is licensed under this
Public License, and include the text of, or the URI or
hyperlink to, this Public License.
2. You may satisfy the conditions in Section 3(a)(1) in any
reasonable manner based on the medium, means, and context in
which You Share the Licensed Material. For example, it may be
reasonable to satisfy the conditions by providing a URI or
hyperlink to a resource that includes the required
information.
3. If requested by the Licensor, You must remove any of the
information required by Section 3(a)(1)(A) to the extent
reasonably practicable.
b. ShareAlike.
In addition to the conditions in Section 3(a), if You Share
Adapted Material You produce, the following conditions also apply.
1. The Adapter's License You apply must be a Creative Commons
license with the same License Elements, this version or
later, or a BY-SA Compatible License.
2. You must include the text of, or the URI or hyperlink to, the
Adapter's License You apply. You may satisfy this condition
in any reasonable manner based on the medium, means, and
context in which You Share Adapted Material.
3. You may not offer or impose any additional or different terms
or conditions on, or apply any Effective Technological
Measures to, Adapted Material that restrict exercise of the
rights granted under the Adapter's License You apply.
Section 4 -- Sui Generis Database Rights.
Where the Licensed Rights include Sui Generis Database Rights that
apply to Your use of the Licensed Material:
a. for the avoidance of doubt, Section 2(a)(1) grants You the right
to extract, reuse, reproduce, and Share all or a substantial
portion of the contents of the database;
b. if You include all or a substantial portion of the database
contents in a database in which You have Sui Generis Database
Rights, then the database in which You have Sui Generis Database
Rights (but not its individual contents) is Adapted Material,
including for purposes of Section 3(b); and
c. You must comply with the conditions in Section 3(a) if You Share
all or a substantial portion of the contents of the database.
For the avoidance of doubt, this Section 4 supplements and does not
replace Your obligations under this Public License where the Licensed
Rights include other Copyright and Similar Rights.
Section 5 -- Disclaimer of Warranties and Limitation of Liability.
a. UNLESS OTHERWISE SEPARATELY UNDERTAKEN BY THE LICENSOR, TO THE
EXTENT POSSIBLE, THE LICENSOR OFFERS THE LICENSED MATERIAL AS-IS
AND AS-AVAILABLE, AND MAKES NO REPRESENTATIONS OR WARRANTIES OF
ANY KIND CONCERNING THE LICENSED MATERIAL, WHETHER EXPRESS,
IMPLIED, STATUTORY, OR OTHER. THIS INCLUDES, WITHOUT LIMITATION,
WARRANTIES OF TITLE, MERCHANTABILITY, FITNESS FOR A PARTICULAR
PURPOSE, NON-INFRINGEMENT, ABSENCE OF LATENT OR OTHER DEFECTS,
ACCURACY, OR THE PRESENCE OR ABSENCE OF ERRORS, WHETHER OR NOT
KNOWN OR DISCOVERABLE. WHERE DISCLAIMERS OF WARRANTIES ARE NOT
ALLOWED IN FULL OR IN PART, THIS DISCLAIMER MAY NOT APPLY TO YOU.
b. TO THE EXTENT POSSIBLE, IN NO EVENT WILL THE LICENSOR BE LIABLE
TO YOU ON ANY LEGAL THEORY (INCLUDING, WITHOUT LIMITATION,
NEGLIGENCE) OR OTHERWISE FOR ANY DIRECT, SPECIAL, INDIRECT,
INCIDENTAL, CONSEQUENTIAL, PUNITIVE, EXEMPLARY, OR OTHER LOSSES,
COSTS, EXPENSES, OR DAMAGES ARISING OUT OF THIS PUBLIC LICENSE OR
USE OF THE LICENSED MATERIAL, EVEN IF THE LICENSOR HAS BEEN
ADVISED OF THE POSSIBILITY OF SUCH LOSSES, COSTS, EXPENSES, OR
DAMAGES. WHERE A LIMITATION OF LIABILITY IS NOT ALLOWED IN FULL OR
IN PART, THIS LIMITATION MAY NOT APPLY TO YOU.
c. The disclaimer of warranties and limitation of liability provided
above shall be interpreted in a manner that, to the extent
possible, most closely approximates an absolute disclaimer and
waiver of all liability.
Section 6 -- Term and Termination.
a. This Public License applies for the term of the Copyright and
Similar Rights licensed here. However, if You fail to comply with
this Public License, then Your rights under this Public License
terminate automatically.
b. Where Your right to use the Licensed Material has terminated under
Section 6(a), it reinstates:
1. automatically as of the date the violation is cured, provided
it is cured within 30 days of Your discovery of the
violation; or
2. upon express reinstatement by the Licensor.
For the avoidance of doubt, this Section 6(b) does not affect any
right the Licensor may have to seek remedies for Your violations
of this Public License.
c. For the avoidance of doubt, the Licensor may also offer the
Licensed Material under separate terms or conditions or stop
distributing the Licensed Material at any time; however, doing so
will not terminate this Public License.
d. Sections 1, 5, 6, 7, and 8 survive termination of this Public
License.
Section 7 -- Other Terms and Conditions.
a. The Licensor shall not be bound by any additional or different
terms or conditions communicated by You unless expressly agreed.
b. Any arrangements, understandings, or agreements regarding the
Licensed Material not stated herein are separate from and
independent of the terms and conditions of this Public License.
Section 8 -- Interpretation.
a. For the avoidance of doubt, this Public License does not, and
shall not be interpreted to, reduce, limit, restrict, or impose
conditions on any use of the Licensed Material that could lawfully
be made without permission under this Public License.
b. To the extent possible, if any provision of this Public License is
deemed unenforceable, it shall be automatically reformed to the
minimum extent necessary to make it enforceable. If the provision
cannot be reformed, it shall be severed from this Public License
without affecting the enforceability of the remaining terms and
conditions.
c. No term or condition of this Public License will be waived and no
failure to comply consented to unless expressly agreed to by the
Licensor.
d. Nothing in this Public License constitutes or may be interpreted
as a limitation upon, or waiver of, any privileges and immunities
that apply to the Licensor or You, including from the legal
processes of any jurisdiction or authority.
=======================================================================
Creative Commons is not a party to its public licenses.
Notwithstanding, Creative Commons may elect to apply one of its public
licenses to material it publishes and in those instances will be
considered the “Licensor.” The text of the Creative Commons public
licenses is dedicated to the public domain under the CC0 Public Domain
Dedication. Except for the limited purpose of indicating that material
is shared under a Creative Commons public license or as otherwise
permitted by the Creative Commons policies published at
creativecommons.org/policies, Creative Commons does not authorize the
use of the trademark "Creative Commons" or any other trademark or logo
of Creative Commons without its prior written consent including,
without limitation, in connection with any unauthorized modifications
to any of its public licenses or any other arrangements,
understandings, or agreements concerning use of licensed material. For
the avoidance of doubt, this paragraph does not form part of the public
licenses.
Creative Commons may be contacted at creativecommons.org.
Lecture 1 (2021-10-04)
1A Reading Notes
Classification of Energy-Related Materials
Passive materials—do not take part in energy conversion e.g. structures in pipelines, turbine blades, oil drills
Active materials—directly take part in energy conversion e.g. solar cells, batteries, catalysts, superconducting magnests
The material and chemical problems for conventional energy systems are mostly well understood and usually associated wit structural and mechanical properties or long standing chemical effects like corrosion:
- fossil fuels
- hydroelectric
- oil from shale and tar
- sands
- coal gasification
- liquefaction
- geothermal energy
- wind power
- bomass conversion
- solar cells
- nuclear reactors
Applications of Energy-Related Materials
High Temperature Materials (and Theoretical Thermodynamic Efficiency)
Thermodynamics indicated that the higher the temperature, the greater the efficiency of heat to work:
where is in kelvin
The first steam engines were only 1% efficient, while modern steam engines are 35% efficient primarily due to improved high-temperature materials.
Early engines made from cast iron while modern engines made from alloys containing nickel, molybdenum, chromium, and silicon, which don’t fail at temperature above 540 C
Modern combustion engines are nearing the limits of metals so new materials that can function at even higher temperatures must be found— particularly intermetallic compounds and ceramics are being developed
Types of Stainless Steel
- Type 304—common; iron, carbon, nickel, and chromium
- Type 316—expensive; iron, carbon, chromium, nickel, molybdenum
Self Quiz 1
What is made of billion year old carbon + water + sprinkling of stardust?
Me
What are the main classifications of materials?
Metals, glass and ceramics,
plastics, elastomers,polymers, composites, and semiconductors[There are] Few Iron Age artefacts left. Why?
They rusted away
What is maens by ‘the micro-structure of a material’?
The very small scale structure of a material which can have strong influence on its physical properties like toughness and ductility and corrosion resistance
What is a ‘micrograph’ of a material?
A picture taken through a microscope
What microscope is used to investage the microstructure of a material down to a 1 micron scale resolution?
Optical Microscope
What microscope is used [to investigate] the microstructure of a material down to a 100 nm scale resolution?
Scanning Electron Microscope
What length scales did you see in the first slide set?
1 mm, 0.5 mm, 1.5 m
What material properties were mentioned in the first slide set?
Hardness, brittleness, melting point, corrosion, density, thermal insulation
Self Quiz 2
What is the effect of lowering the temperature of rubber?
Makes it more brittle, much less elastic and flexible
What material properties were mentioned in the second slide set?
Young’s modulus, specific heat, coefficient of thermal expansion
Lecture 2
Properties of the Classes
Metals
- Ductile (yields before fracture)
- High UFS (Ultimate Fracture Stress) in tension and compression
- Hard
- Tough
- High melting point
- High electric and thermal conductivity
Ceramics and Glasses
- Brittle — elastic to failure, no yield
- Hard (harder than metals)
- Low UFS under tension
- High UFS under compression
- Not tough
- High melting points
- Do not burn as oxide ceramics are already oxides
- Chemically resistant
- Poor thermal and electric conductivity
- Wide range of magnetic and dielectric behaviours
Polymers
Organic—as in organic chemistry (i.e. carbon based)
Ductile
Low UFS in tension and compression
Not hard
Reasonably tough
Low threshold temperature to charring and combustion in air or pure oxygen
Low electrical and thermal conductivity
- There are some electrically conductive polymers
Composites
Composed of 2 or more materials on any scale from atomic to mm scale to produce properties that cannot be obtained in a single material
- Larger scale mixes of materials may be called ‘multimaterial’
Material propertes depends on what its made of
Terms
Organic vs Inorganic Materials
- Organic materials are carbon based
- From chemistry, organic compounds are ones with a C-H bond
- Inorganic compounds do not contain the C-H bond
Crystalline vs Non-Crystalline Materials
Most things are crystalline
- Ice
- Sugar
- Salt
- Metals
- Ceramics
Glasses are non-crystalline
Material Properties
Density
Density if quoted at STP (standard temperature and pressure— K and Pa)
Metals, ceramics, and glasses are high density materials
Polymers are low density
Composites span a wide range of density as it depends on the materials it is composed of
e.g. composites with a metal matrix will have a much higher density than those with a polymer matrix
Melting Points
Measured at standard pressure and in an intert atmosphere (e.g. with Nitrogen, Argon, etc)
- Diamond and graphite will survive up to 4000 C in an inert atmosphere but would burn at around 1000 C in oxygen
High melting points -> high chemical bond strength
Corrosion
It’s not just metals that corrode
Polymers
- UV degradation
- Water absorption can occur in degraded polymers
Glass
- Leaching
- Sodium ions can leave the glass when covered in water. If the water stays, the high pH water can damage the class
Self Quiz
Consolidation Questions 1
- Metal
- Titanium
- Polymer
- Polyester
- Ceramic
- Alumino-silicate
- Composite
- GFRP or CFRP
- Metal
- Aluiminium alloy
- Metal
- Aluiminium alloy
- Polymer
- Acrylic
- Ceramimc
- Glass
- Composite
- Concrete
Consolidation Questions 2
CB
Polymers
Introduction to Polymers
There are 3 types of polymers:
thermoplastics
No cross links between chains. The lack of cross links allows recycling of polymers by heating it above the glass transition material, , lowering the viscosity.
An example of thermoplastics is PET, used in water bottles
thermosets

has lots of cross-links between chains, making it more rigid. Heating does not lower its viscosity making them much harder/impossible to recycle.
and example of thermosets is melamine formaldehyde, used on kitchen tabletops
elastomers

has some cross links and a lot of folding of chains
Latex is an example of an elastomer
Polymers are relatively new materials, lightweight, durable, flammable, and degraded by UV light. They are made of long carbon-carbon chains.
Stress-Strain Curve of Polymers

Industrially Important Polymers
The worldwide production of polymers in 2019 was tonnes and the majority is from just 5 polymers:
- Polyethylene (PE) — wire insulation, flexible tubing, squeezy bottles
- Polypropyene (PP) — carpet fibres, ropes, liquid containers, pipes, chairs in Shoreham Academy
- Polyvinyl chloride (PVC) — bottles, hoses, pipes, valves, wire insulation, toys
- Polystyrene (PS) — packaging foam, egg cartons, lighting panels
- Polyethylene terephthalate (PET) — carbonated drinks bottles
All of these materials are low cost.
Thermoplastics
The simplest polymer is poly(ethene):
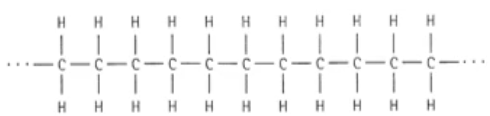
When 2 polymer chains get close together, Van der Waals (vdw) forces keep them together. vdw forces are very weak, much weaker than the covalent bonds inside the polymer.
Stress Strain Curve
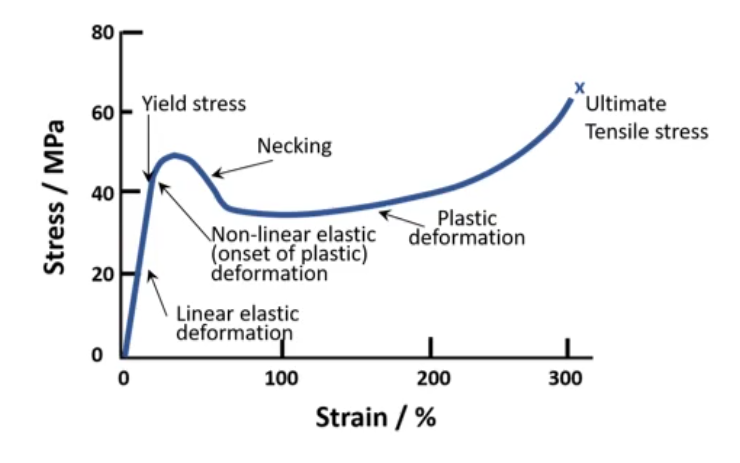
- During linear deformation, the carbon chains are strethed.
- At yield stress, the carbon chains get untangled and slide past eache other.
- Necking initially allows the chains to slide at lower stress.
- As the chains pull, align, and get closer, the vdw forces get stronger and more stress is required to fracture.
Crystalline and Amorphous/Glassy Solids (Heating and Cooling)
Amorphous Thermoplastics
- As you heat above , the chains get easier to move past each other.
- It is known as an amorphous supercooled liquid.
- There is not really a melting point are there are no crystals, but is the point where the chains are easy to move
Crystalline Polymers
- The glass transition point does not exist for crystalline polymers
- The solid is difficult to deform below and is not ductile
- Above the chains are very easy to move past each other
Semi-Crystalline
- Below , only local movements in chains are possible, so the material is less ductile. The solid crystalline regions makes it difficult to move the chains.
- Between and , the glassy chains are easier to move but the crystalline regions remain difficult
- Above the chains easily move past each other
Specific Volume vs Temperature

Path ABCD
- a-b — Start cooling the true liquid
- b-c — At the freezing point, , the true liquid freezes diretly to a crystalline solid
- c-d — The crystalline solid cools t room temperature as the temperature is lowered
Path ABEF
a-b — start cooling the true liquid
b — at the freezing point nothing freezes
b-e — the liquid becomes supercooled and contracts and becomes more viscous as the temperature decreases.
The supercooled liquid region is between and
Supercooling requires you to cool the sample quicker than you would for path ABCD
e — is reached and the supercooled liquid sets to a amorphous solid
e-f — the amorphous solid cools from room temperature and contract as the temperature is lowered
Relative Molar Mass and Degree of Polymerisation
- Number Average RMM —
- Weight Average RMM —
- Degree of polymerisation — and
where
- is the RMM of the chain
- is the fraction of the polymer that is composed of that chain by number/quantity
- is the fraction of the polymer that is composed of that chain by mass/weight
- is the RMM of the monomer from which the polymer was made
Making Polymers
There are two ways to make polymers:
Elastic Deformaion
Elastic deformation is deformation where the material will return to original shape after the applied stresses are removed.
Elastic deformation is the first type of deformation that happens when stresses are applied to a material and is represented by the straight line at the beginning of a stress-strain curve.
Modulus of Resillience ()
This is the area under the elastic portion of a stress-strain graph of a material.
Plastic Deformation
Toughness (Absorbing Energy Through Plastic Deformation)
- The toughness of a material is its ability to absorb energy through plastic deformation without fracturing
- The material toughness of a ductile material can be determined by finding the area under its stress-strain curve (e.g. by integrating the graph)
- Brittle materials like ceramics and glasses exhibit no material toughness
- Ductile materials have a possibility of achieving large material toughness
Ductility measures how much something deforms plastically before fracture, but just because a material is ductile does not make it tough.
The key to high material toughness is a good combination of large ultimate fracture stress and large ductility.
The unit of toughness is energy per unit volume as toughness can be mathematically expressed as:
A metal may have satisfactory toughness under static loads but fail under dynamic loads or impact
This may be caused by the fact that ductility and toughness usually decrease as rate of loading increases.
Ductility and toughness decreasee with temperature
Notches in the material affect the distribution of stress in the material, potentially changing it from a uniaxial stress to multiaxial stress
Charpy Impact Test
Measures material toughness by determining the amount of energy absorbed during fracture.
It works by essentially dropping a hammer into a sample whose dimensions are standardized (usually either by BSI or ISO) and measuring how high the hammer goes up on the other side, after it breaks the material
The height of the hammer after impact will tell you how much enery is left in it, and therefore how much has been aborbed by the now broken sample.
Under a microscope, more ductile fractures appear fibrous or dull, whereas less ductile surfaces have granular or shiny surface texture.A
The charpy test has a couple issues:
- Results are prone to scatter as it is difficult to achieve a perfectly shaped notch
- Temperature has to be strictly controlled since it affects a material’s ductility
The setup of a charpy impact test
- Sample is made to standardized dimensions, with a notch
- Sample is placed on support
- A very heavy hammer pendulum of mass is dropped from rest at to swing about a pivot, reaching vertically below the pivot.
If no sample is in place then the hammer will swing back up on the other side to a height of where theoretically
With a sample placed vertically below, some of the is transferred to the sample to bend and (usually) break the sample.
If breaks the sample, it will swing up to the other side, where its max height, can be used to calculate how much energy was used to break the sample:
Where is acceleration due to gravity.
Ductility
Ductility is the plastic deformation a material withstands before fracture.
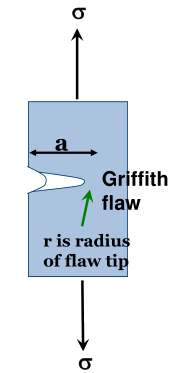
Griffith Surface Flaws
These flaws vary in size and shape. They limit the ability of any material, brittle or ductile, to withstand tensile stresses as they concentrate the tensile forces applied to a smaller area.
The stress at the tip of the flaw:
For deep ( is large) or thin ( is small) the stress is magnified and, if it exceeds the UFS in a brittle material, the flaw will grow into a crack, resulting in the brittle material fracturing.
However in a ductile material, the tip of the flaw can heal, reducing and increasing . This is due to the chemical structure of ductile materials like metals.
Stress Intensity Factor
Stress Intesity Factor, :
where:
- is the geometry factor (1 would represent an infinite width sample, and 0 a 0 width sample)
- is applied tensile strength
- is flaw depth
Fracture Toughness
![An example sample for testing fracture toughenss. From: https://www.researchgate.net/figure/Compact-tension-sample-geometry-used-for-fracture-toughness-measurement_fig2_340037774 [accessed 8 Nov, 2021]](./images/Compact-tension-sample-geometry-used-for-fracture-toughness-measurement.png)
The value of that causes the notch to grow and cause fractures. This is value is known as the fracture toughness, .
At low thicknesses fracture toughness depends on thickness but as thickness increases, decreases to the constant value, the plane strain fracture toughness, .
Composites
Composites are made of two or more materials, which when combined together, at up to a milimetre scale, have superior properties to their parent materials.
Composites tend to be 2-phase: a dispersed phase in a matrix. The disepersed phase tends to be fibres (large aspect ratio) or particles (low aspect ratio) which are embedded in a matrix, which are often resins.
Composite properites are affected by the dispersed phase geometry:
- Shape
- Size
- Distribution
- Relative orientation (for fibres)
Rule of Mixtures
lies between the arithmetic mean (upper limit):
and the geometric mean (lower limit):
Where , , are the Young’s moduluses of the composite, matrix, and particles, respectively, and and are the volume of the matrix and particles, respectively.
Particle Reinforced Composites
Applications of Composites
Tungsten Carbide
Cobalt for Cutting Tools
The Tungest Carbide (WC) particle are a truly brittle ceramic. They are very hard but the brittleness means they are easy to break.
The solution is to hold small WC particles in a ducitle metal matrix. In this case it is Cobalt (Co).
This way, crack in one WC particle does not necessarily mean other particles are broken, meaning the cutting tool overall still works.
Another advantage of this composite is that WC is not very thermally conductive and has a high melting point, which allows it to work well the environment it’s in.
Resin Bonded Alumina for
Sanding Disks
This is another example of brittle but hard ceramics being put in a ductile matrix. In this case it’s a resin.
It follows the same idea—separating the ceramics into small particles means the particles can break and the product still works overall, as there are thousands of particles which are not broken.Fibre Reinforced Composites
Specific Property
Specific Property of a composite is a property divided by density of composite. Here are some examples of specific properties:
- Specific ultimate tensile stress
- Specific Young’s modulus/stiffness
Influence of the Fibres
Depends on:
- Fibre type
- Fibre length and diameter
- Fibre orientation
- Strength of bond between fibre and matrix
Stress Strain Graph of a Fibre Reinforced Composite
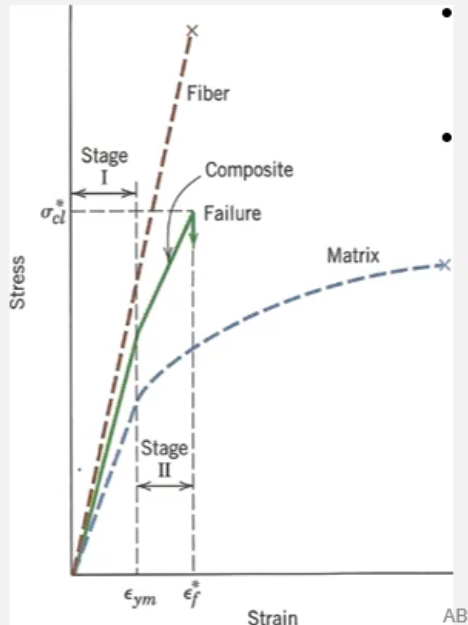
Note that the composite fails at the same strain as the fibres but yields at the same strain as the polymer matrix.
The elastic behaviour of the composite before yielding is dependent on the strength of the chemical bonds between the surface of the fibre and matrix.
Mechanical Performance of a Fibre Reinforced Composite
Stress/strain behaviour of fibre
Stress/strain behaviour of matrix
Fibre volume fraction
Applied stress direction
Longitudinal is along direction of fibres, transverse is 90to direction.
Fibre composites tend to be much much weaker in transverse direction:
Composite Longitudinal UTS Transverse UTS GF/PET 700 20 CF/Epoxy 1000 35 Kevlar/Epoxy 1200 20 (All units in MPa)
Thermal Properties of Materials
Specific Heat Capacity
How much heat energy is required to raise the temperature of a body by one unit:
where is specific heat capacity.
It is measured at a constant pressure, usually Pa.
Molar Heat Capacity
What is a mole?
The mole (symbol: mol) is the base unit of amount of substance in the International System of Units (SI). It is defined as exactly elementary entities (“particles”)
How Much Does a mol of
Something weigh?
A mol of an element weighs its relative atomic mass () but in grams. For example, Carbon-12 has an of 12 (as it’s made of 6 neutrons, 6 protons, and 6 electrons which have negligible mass) so a mol of Carbon-12 has a mass of 12 g.
Thermal Expansion
Origin of Thermal Expansion
All atomic bonds vibrate, on the magnitude of gigahertz. The bonds vibrate about a mean positoin and the vibration is a simple harmonic motion.
From the graph below you can see that as energy (in the form of heat) is supplied to the bonds, the amplitude of the vibrations get larger and larger. You can also see the mean position of the bond gets further and further away, meaning the volume of the material also is increasing. The mean position of the bond is what dictates the volume, as this means the inter-atomic separation increases.
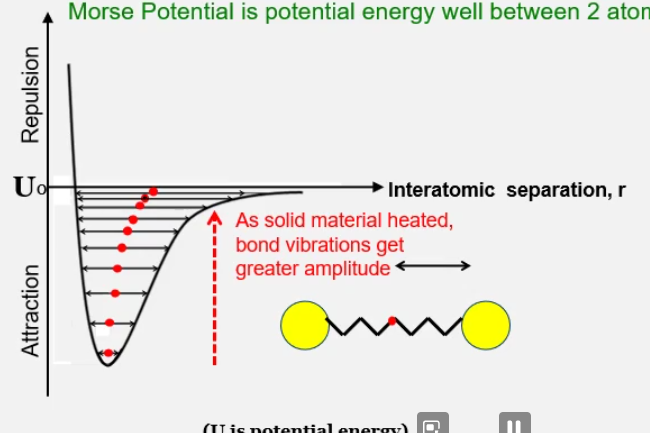
Morse potential is the energy well between 2 bonded atoms. The graph is asymmetric due to the repulsion experienced by atoms as they apporach.
Linear Coefficient of Thermal Expansion
where is the sample length.
Example 1
A 1 m long bar of aluminium metal cools in the solid state from 660 C
to 25 C.
Calculate the length of the bar after it cools down, given
K.
Calculate the length of the bar after it cools down, given K.
Linear Thermal Expansion and Isotropism
Since isotropic solids have the same properties in all directions, you can say that for an isotropic solid:
Reasons to Care About Thermal Expansion
- A coating on a material may fail if the thermal expansion coefficients do not match
- A brittle material may thermally shock and fracture due to thermal expansion mismatch between the ouside and inside, especially if the material is not very thermally conductive
Thermal Conductivity
Thermal conductivity is the rate at which heat power is transferred through a material.
where is heat power, is area of the surface, is the temperature gradient, and is the thermal conductivity constant.
Origin of Thermal
Conductivity
Heat is transferred through materials by electrons (and partially by atomic vibrations)
Metals have high thermal conductivity as their delocalised ‘sea’ electrons are about to move about easily. This makes them excellent conductors of heat and electricity.
Ceramics, glasses, and polymers do not have delocalised electrons and are therefore poor conductors of heat and electricity (they are insulators).
Polymer foams are even better insulators because they have holes which lowers their density.
Chemical Bonding of Materials
Chemical bonds are what holds a material together in solid state. There are 5 main types of bonds:
| Type | Dissociation energy |
|---|---|
| Ionic | 600 to 1500 |
| Covalent | 300 to 1200 |
| Metallic | 100 to 800 |
| Hydrogen | 4 to 23 |
| vdw | 0.4 to 4 |
The dissociation energy is the energy required to break the bond, or the strength of the bond.
Materials and their Properties and Bonding
Ceramics and Glasses
Ceramics and glasses are composed of mixed ionic and covalent bonding. Their strong and rigid bonds have no ability to slide past each other. This makes the materials brittle.
Metals
Metals are based on metallic bonding (woah). This type of bonding does allow for ions to slide past each other, making metals ductile.
Polymers
Polymer chains made of C-C covalent bonds are strong, like those found in ceramics.
However, in thermoplastics polymers, the materials can yield by having the chains untangle and then align, as the chains slide past each other. This means that stronger bonds between polymer chains means a higher yield stress in thermoplastic polymers.
Crystallisation of Materials
Atomic Arrangement
No order
Short range order
Silica glasses have short range order on the atomic scale. They are composed of regular SiO units which all have the same bond length and bond angles.
However, these units bond together irregularly, which results in different length chemical bonds and angles between the units, meaning they do not have any long range order.
Long range order
Cubic Unit Cells
Lattice Parameter — One side of a unit cell
The lattice parameter can be different for each side of a cell.
Simple cubic unit (SC):

Lattice Parameter = 2r
Face centred cubic (FCC)

Body centred cubic (BCC)
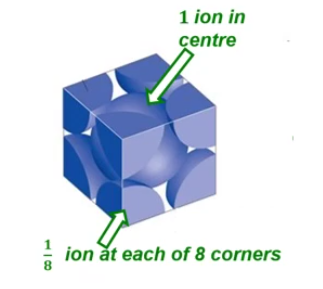
Packing Factor
Theoretical Density
Polymorphism
Example of a polymorphic solid-state phase transfomration of iron at 1185 K and 1 atm:
Below 1185 K and at 1 atm, only BCC exists. Above 1185 K and at 1 atm, only FCC exists.
Points, Directions, Planes in a Cubic Unit Cell

Slip Systems in Metals
Metal ions lying in close-packed planes and directions move more easily, increasing ductility. The combination of a close packed plane and direction is called a slip system.
A close packed direction is where ions touch all the way along the direction.
A close packed plane is where ions touch all the way on a plane.
FCC metal ductility is mainly controlled by the (111) slip plane

X-Ray Diffraction (Bragg’s Law)
The wavelength of x-rays, , is roughly equal to the distance, , between atom/ion layers. This allows x-rays to probe for via Bragg’s Equation:

Requirements for the x-rays:
- Monochromatic
- Coherent (phase difference of where n is any integer)
- Parallel with each other
The incoming x-rays 1 and 2 strike the rows of ions in the crystal and are diffracted, which can be considered reflection at the atomic level. The angle of incidence equals the angle of reflection.
The outgoing x-rays 1 and 2 are coherent only if the extra path travelled by ray 2, is any multiple, , of . Or:
This is Bragg’s Law.
Metals
Defects on the Atomic Scale
Defects on the atomic scale have a significant effect on yield stress, ultimate tensile stress, and ultimate fracture stress.
The yield stress of a real metal(-alloy) is much lower than the theoretical yield stress for the perfect metal(-alloy) crystal. This difference is because of the defects in the metal, particularly dislocations, as the dislocations allow the ions to slide past each other at much lower yield stresses.
The 5 types of defects are:
- Grain boundaries
- Vacancies (missing ion)
- Dislocations (missing row of ions)
- Impurity ions
- Crystalline includison

Dislocation Movement vs. Simple Sliding
The layers of ions in a crystalline metal could simply over each other:

However, the stress required for simple sliding is much higher than the stress required to move a dislocation. This is because dislocation motion is successive sliding of the partial plane of ions under applied shear stress (black arrow). The vacancy in the slip plane (yellow arrow) moves in steps in sequence from left to right.
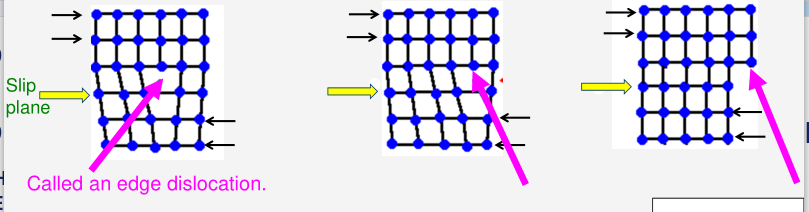
If there are no dislocations then plastic deformation is delayed to a higher applied stress, meaning the yield stress of the metal would be much higher.
Dislocations move more easily on specific planes and in specific directions called the slip planes and slip directions which make up what is known as the slip system.
There are a very large amount of dislocations in metals and alloys. Dislocation density is expressed as total length of dislocations per unit volume.
Single Crystal Metals

Normally when a molten metal is cooled to a solid, then lots of tiny crystals (grains) grow in different directions until they impinge. The grain boundaries are a source of mechanical weakness.
A single crystal metal is one for which the casting is cooled to form just one giant crystal:
- The molten metal is cast into a mould
- At the very base of the mould, the temperature is dropped and the alloy crystallises into many little crystals
- The crystals grow upwars through the liquid and meet a spiral tube and are constricted
- This tube only allows one crystal to grow through the spiral and then into the main mould
Polycrystalline Metals
Most normal metals you see everyday are polycrystalline.

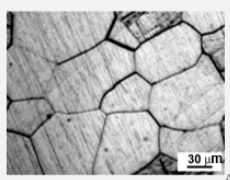
Elastic and Plastic Strain in Metals
When you apply a tensile stress to a mteal, this will produce a shear stress in any part of the metallic lattice that is not parallel or perpendicular to the applied stress. Under the action of shear stress, the metallic lattice will tend to experience a combination of elastic strain and plastic strain:

Raising the Yield Stress of a Metal
There are 4 main ways to raise the yield stress of a metal:
- Make a solid-solution—by metal alloying or atomic addition
- Precipitate crystalline inclusions—by metal alloying or atomic additions and then heat treatment
- Work-harden — by processing and/or cold-working
- Decrease the grain size — by processing and/or heat-treatment
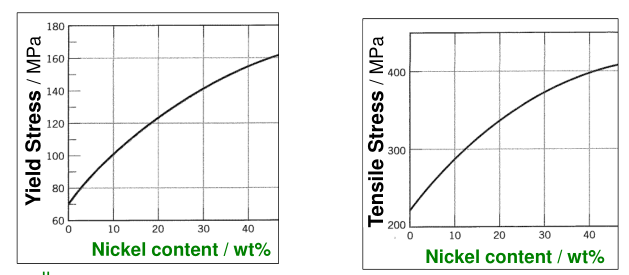
Make a Solid-Solution
Adding an alloying element, B, to the host, A, forms a solid-solution as the ions or atoms of B dissolve in A.
The impurity particles of B are a different size from the particles of A, distorting the metal lattice. The larger the difference in radii of the particles, the bigger the distortion.

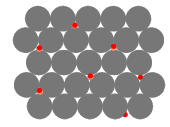
The particles of B tend to diffuse to dislocations and immobilise them. This is why alloying increases the yield stress.
Impurity particles generate lattice strain in the structure too:
- Smaller particles introduce a compressive strain in the surrounding lattice
- Larger particles introduce a tensile strain in the surrounding lattice
Precipitating Crystalline Inclusions
When adding an element, B, to a host, A, exceeds the solubility, the result is the formation of a solid-solution with a fixed ratio of B to A, but also precipitated crystals of a different ratio of B to A.

Crystalline inclusions are really difficult to shear, especially if they are small, numerous, and have high Vickers’ hardness. This slows down dislocation movement, increasing yield stress.
Work-Hardening and Cold Working
We can use room temperature deformation to increase the number of dislocations present in a metal. As the % cold-work (%CW) is increased, the number of dislocations present also increases:
where is the initial cross sectional area and is the final cross sectional area.
A carefully prepared sample has a dislocation density, of around mm mm, whereas for a heavily deformed sample it is around .
A high density of dislocations means they are more likely to get entangled with each other, making it harder for dislocations to move. Therefore as increases, yield stress does too.
Decreasing the Grain Size
- Most metals are polycrystalline with many grains.
- Different grains will have a different crystal orientation.
- Grains impede dislocation motion
As you decrease grain size, you get more grain boundaries which basically creates more barriers to prevent slip.
This is because a dislocation would have to change orientation across a grain boundary and “ionic disorder in the grain boundary results in discontinuity of slip” (A.B Seddon, University of Nottingham 2020) (I think that’s repeating it but it said it on the slideshow sooo…).
So for any given metal, the fine grained is harder and has greater yield stress than the coarse grained version of it.
Hall Petch Equation
where is the grain size and and are material constants.
Therefore a plot of against would results in a straight line.
Heat Treatment of Metals
These processes are to change a material’s mechanical properties, not change its shape.
Phase Diagrams
Here is an example of a two component phase diagram with a familiar system:
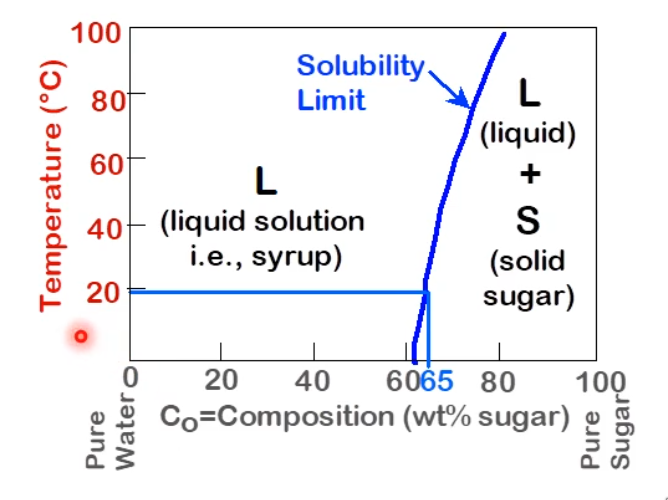
The component in this case are sugar and water, but not syrup.
A phase is a chemically and physically distinct species as we can have a change in phase that goes from solid to solid.
The solubility limit is the maximum concentration for which only a solution occurs. In the case of this system, thee limit increases with temperature.
Here is a generic phase diagram for a generic A-B system:

- L - liquid
- — a solid phase but still a solution. B can dissolve into A
- — a solid phase but still a solution. A can dissolve into B
Annealing
Annealing is a process by which a component is heated to remove the effects of cold work.
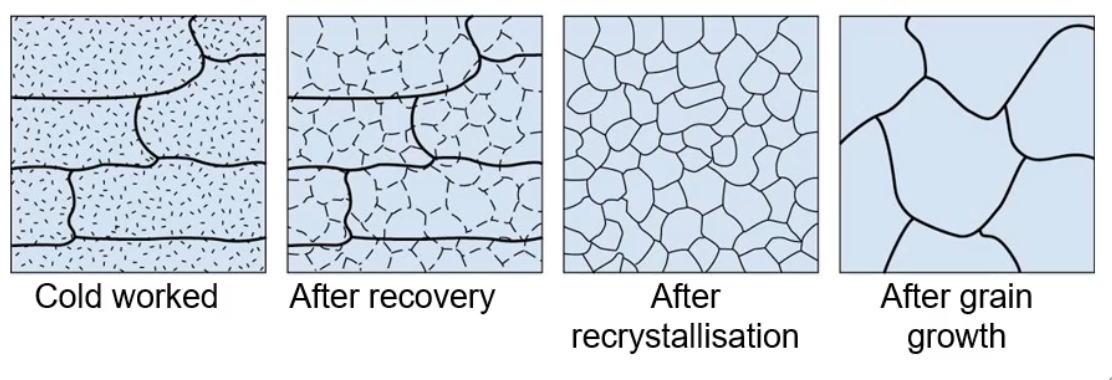
These are diffusional processes and only occur at high temperatures. The driver for diffusion is the removal of high energy defects from the system.
Diffusion
Diffusion is atomic or ionic movement down a concentration gradient.
Solid State Diffusion

Solid state diffusion is the stepwise migration (march) of atoms or ions through a lattice, from site to site.
In order for this to happen, there must an adjacent vacant site. The diffusion particle must also have sufficient thermal energy to ‘jump’ to the new site.
Vacancy Diffusion (Diffusion of Metal Ions)

Interstitial DIffusion (Diffusion of Small, Non-Metallic Particles)

The Math(s) of Diffusion
Diffusion is time dependent.
For steady state diffusion, Fick’s 1st Law holds:
where is the flux, is the concentration gradient, and is the constant of proportionality known as the diffusion coefficient.
is constant for a particular metal at a particular temperature. The fluxis the number of atoms or ions moving per second through a cross sectional area.
Things that Affect the Speed of Diffusion
size of the diffusion species — smaller species results in faster diffusion
temperature — more thermal energy allows more particles to have enough energy to make the ‘jump’
host lattice
- simple cubic — 52% occupancy of ions
- body centered cubic — 68% occupancy of ions
- face centered cubic — 74% occupancy of ions
Diffusion is faster in a BCC host than in an FCC host for iron ions in an iron host and also for carbon atoms diffusing into an iron host. However this is not always the case.
Influence of Temperature on Diffusion (Arrhenius Equation)
You can apply the Arrhenius equation for all thermally activated diffusion:
where is the diffusion coefficient, is the frequency factor, is the activation energy, is the ideal gas constant (8.31 J k mol).
You can find the diffusion distance, , with the following equation:
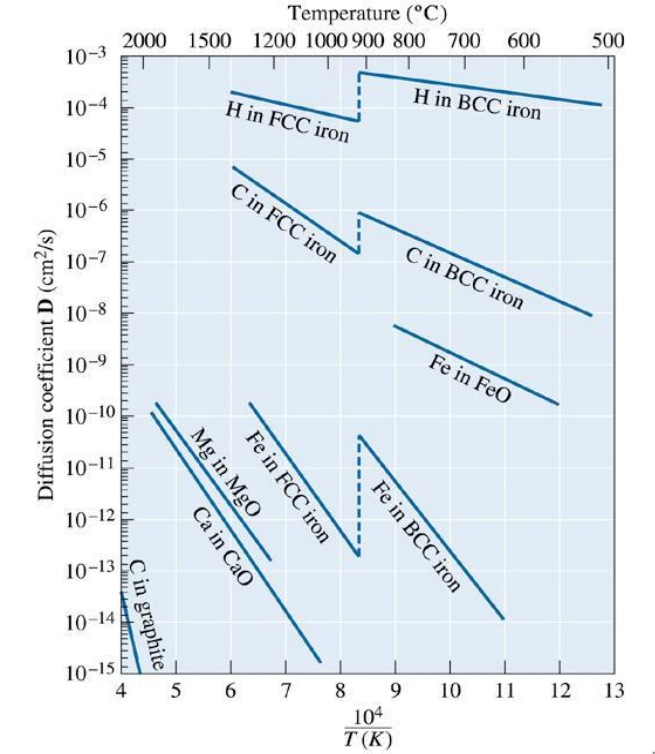
Materials in Sustainable Transport
- Concerns over use of fossil fuels, climate change
- Const of energy
- Energy use in making and moving vehicles
- Rising energy prices mean cost of fuel is comparable to cost of car
- 1/4 of energy used in UK is to transport goods and people
- Legislation and voluntary targets set by EU to improve fuel efficiency
- In 2015 average CO2 emmisions as 130 g / km
- Engine powerhas gone up significantly from 2001 to 2018 (~30%) yet engine displracement has gone down ~10% and CO2 emissions down ~18% while weight has gone up ~10%
Is the car emissions reduction target significant?
Overall CO2 emissions in 2016 is 466 Megatonnes.
Does a reduction from 130 g / km to 95 g / km (a 35 g/km reduction) make a significant difference?
There are 33 million registered cars in the uk.
If they average around 8000 miles each (~13000 km) per year that’s a ~15 Megatonne reduction, or about 3% of the annual C02 emmissions, a significant reduction.
Materials in Cars
- Most of the energy used by cars is during driving (71%)
- This means the mass of the vehicle has a great effect on its emmissions across a lifetime
- The body, suspension, drivetrain, and interior all contribute roughly a quarter to the mass of the car
- However, the mass of cars are increasing
Material Substitution
The material will likely have performance requirements:
- It may need to be a physical size
- It may need to operate at certain temperatures
- It may need to bear a certain load
The component mustalso be designed for convenient manufacturing, assembly, servicing, disposal, remanufacturing and/or disassembly
Case Study — 2012 Honda Accord
- Body — opted to stay with steel — aluminium intense and multi-material approaches were both rejected due to higher costs and limitations in manufacturing and assembly. Recyclability was also noted as an issue due to different grades of aluminium needing to be separated at end of life.
- Doors and bonnets — move to aluminium from steel — more costly but the mass savings made this option worth it
- Wiring — aluminium to copper — lower mass for same conductivity, copper is more expensive (I think)
- Seats — steel to composites or magnesium structural components — very high weight savings
Choosing a Material
Glossary
- liquidus - for a system of more than one component, the liquidus is the lowest temperature at which the whole system is all in the liquid state.
- solidus - for a system of more than one component, the solidus is the highest temperature at which the whole system is still in the solid state
page rendered by gronk